Phys9ii
THERMAL PHYSICS: part 2
HEAT AND TEMPERATURE
Heat- amt of thermal energy that flows from high to low temp
Temperature-degree of hotness or coldness ; a measure of av.KE of molecules of a body
temp measured by thermometer, usually in degrees Celsius (°C), uses a physical property which changes continuously w/ temp (thermometric property)
Temperature Scale
2 requirements needed in defining a temp scale
-a thermometric property: any property of a material which varies continuously w/ temp, eg vol of Hg/alcohol, pressure of gas, R of a metal, thermoelectric emf in a thermocouple etc
-2 fixed pts (upper & lower fixed pts): most common- freezing pt (0°C)& boiling pt (100°C) of pure water (at normal atmospheric pressure, 760 mm/Hg) (both 0°C and 100°C are arbitrary)
Constructing a temp scale
Take a thermometric subs > choose 2 standard degrees of hotness which are easily obtainable and reproducible (fixed pts) > values of physical property recorded at these 2 temp > temp range divided into a # of equal divisions
Centigrade Scale [based on ice pt (lower fixed pt) and steam pt (upper fixed pt)]
Ice pt (lower fixed pt- temp of pure melting ice, pressure = 1 atm, assigned value of 0°C)
-lower part of thermometer immersed in a funnel w/ pure melting ice
-ice shavings used (ensures good contact)
-Hg level remains steady > 0°C
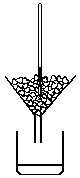
Steam pt (upper fixed pt- temp of dry steam from water boiling, pressure = 1 atm, assigned value of 100°C)
-lower thermometer into apparatus, bulb just above boiling water
-manometer included to ensure pressure inside apparatus is = 1 atm
-Hg level remains steady > 100°C

dist between ice pt and steam pt divided into 100 equal parts (assumes mercury expands linearly to temp)
Calculating temp on Centigrade scale
θ = _Lθ - L0_ x 100
L100 - L0
(θ = temp in °C, Lθ = length of Hg at θ°C,
L0 = length of Hg at ice pt, L100 = length of Hg at steam pt)
The Kelvin (Absolute) Scale
-base on one fixed pt: the triple pt of water: temp where saturated water vapour, pure water ad ice all coexist in equilibrium
-0 K = absolute or true 0 of temp: where all molecules have zero-point energy
-Celsius scale reconciles Centigrade and Kelvin scales: θ /°C =T/K - 273.15, θ = temp in celsius > 1 kelvin = 1 degree celsius
Thermometric liquids
2 liquids used in liquid-in-glass thermometers: mercury and alcohol
Mercury
Advantages
-metal: good conductor of heat > whole liquid reaches temp of surroundings quickly > quick response to temp changes
-doesnt wet (cling to sides of) the tube
-high bp(357°C), can measure high temp
-expands uniformly
-has visible meniscus
Disadvantages
-poisonous
-expansivity is fairly low
-relatively expensive
-high freezing pt (-39°C)
Alcohol
Advantages
-expands uniformly
-low freezing pt (-115°C)
-large expansivity (6x more than Hg)
-cheap
-safe liquid
Disadvantages
-wets the tube
-low bp (78°C)
-doesnt react quickly to changes in temp
-colourless
Labratory thermometer
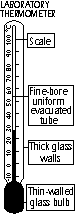
Thick glass walls
-acts as magnifying glass > easy reading & stem won't break easily
Fine-bore uniform evacuated tube
-fine: small change in temp > noticeable movement (more sensitive)
-uniform: even expansion of liquid
Scale
-usually in 1° divisions but can read to 0.1°C on very long, fine-bore tubes
Thin-walled glass bulb
-quick conduction of heat through glass (poor conductor of heat- quick response)
Bulb
-small, contains small amt of liquid- more responsive (too little-heat quickly lost, too much- less responsive)
small- portable, cheap to produce, gives direct reading-convenient (but not accurate-glass expands)
Clinical thermometer (sterilesed in antiseptic, not hot water)
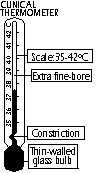
Constriction(tube v.v.thin)
-ensures max temp recorded, breaks mercury thread from flowing back into bulb (mercury expands pass it)
Extra fine-bore
-improve sensitivity: large change in length for small change in temp
Thin-walled glass bulb
-thinner than normal, v.rapid heat conduction (quick response)
Scale (35-42°C, body temp 36.9°C)
-short range: greater accuracy & stem can be made reasonably short
Cross section of stem: pear / oval / triangular-shaped, acts as magnifying glass in one direction
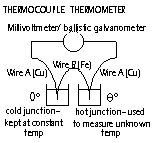
Thermocouple thermometers
-consists of 2 wires of diff metals: Cu + Fe/ constantine (R ~about at any temp) whose ends are joined together to form 2 junctions
-if the two junctions are at diff temp a small emf is produced = thermoelectric effect, greater difference in temp > greater emf
E = Δθ (E = induced, Δθ = temp difference bet 2 junctions)
Advantages
-can measure v.large temp range: -200 to 1500°C, using suitable wires
-wire junctions v.small, can measure temp at a pt
-v.responsive to rapidly changing temp due to low thermal capacity due to small mass & because metals are good conductors of heat
thermopile: thermocouples joined in series if a larger voltage is needed- v.sensitive to heat
Heat: form of energy, joule J
Heat supplied to water converted to internal energy (KE + PE) of water molecules, heat- a measure of internal energy
Solid, liquid, gas: heated > KE ^, PE ^ (slightly-expansion), mp + bp: heated > KE-constant, PE ^ (molecules more disordered)
Heat capacity, C: amt of heat required to raise temp of a body by 1°C, (J/K, J/°C), depends on mass & material
C = Q/Δθ (Q = energy supplied, Δθ = change in temp)
Specific heat capacity, c: amt of heat required to raise temp of 1kg of a subs by 1°C, (Jkg-1K-1, Jkg-1°C-1), heat capacity per unit mass
c = Q/mΔθ (Q = energy supplied, Δθ = change in temp, m = mass of object)
(water: high s.heat capacity, used as cooling agent, metals lower s.heat capacity)
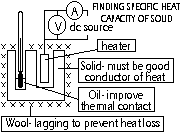
Record: mass of block, initial temp, time heater on, V & I (during heating), final temp (max reading after power off)
Assumption- heat provided by heater entirely absorbed by solid block (proper lagging needed)
VIt = mcΔθ
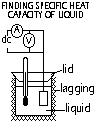
Heat supplied = heat absorbed by (liquid + container)
VIt = mlclΔθ + mcccΔθ (assume no heat loss, good lagging)
Melting, solidification, boiling, condensation
-energy transfer w/o temp change due to latent heat of fusion (melting, solidification) or vaporisation (boiling, condensing)
Melting: solid > liquid state when heat is supplied, pure subs; mp-fixed temp
Latent heat of fusion: heat absorbed w/o a change in temp during melting(latent = hidden)
Boiling: pure liquid on heating changes to vapour at fixed/ constant temp = bp
Latent heat of vaporisation: heat absorbed w/o a change in temp during boiling()
Melting & boiling: energy absorbed to break bonds > molecules move more freely (change state)& boiling-push away air molecules
Solidification: reverse of melting: liquid > solid, pure subs freeze at temp equal to mp (fp = mp)
Condensation: reverse of boiling- vapour cooled > liquid at same constant temp (cp = bp)
Solidification & condensation: energy released in bond formation (more ordered structure)
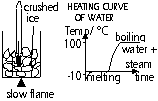
To find mp or bp:
plot temp against time graph of (s) > (l) (melting) or (l) > (g) (boiling): straight line = mp or bp
To find fp or cp:
plot temp against time graph of (l) > (s) (freezing) or (g) > (l) (condensation): straight line = fp or cp
(temp only changes when subs in same state)
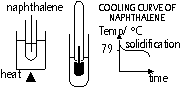
Impurities(salt, antifreeze):
lower fp > prevent freezing over & formation of ice, salt: make ice cool objects < 0°C, raise bp
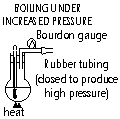
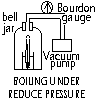
Pressure; pressure ^ :
mp decrease, bp ^ (decreased bp: use less energy to rid unwanted water, bp ^ : cook food faster)
Latent heat of fusion, Lf(J)- heat energy required to change solid to liquid state w/o change in temp (specific,  f = per unit mass)
f = per unit mass)
Lf =  f x m
f x m
Measure 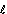 f of ice
f of ice
Put warmed water in a Cu calorimeter > find mwater > record T1, room temp + 10°C > add ice 1 at a time > 1 melt > add another > record T2, room temp -10°C (compensate for heat loss > reduce error) > find mice
Assuming negligible heat loss:
heat loss by water = heat gained by ice to become water + heat gained by melted ice to raise temp form 0°C to T2
mwatercΔθ1 = mice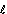 f + micecΔθ2 (Δθ2 = T2)
f + micecΔθ2 (Δθ2 = T2)
Latent heat of vaporisation, Lv (J)- heat energy required to change liquid to vapour state w/o change in temp (specific 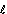 v = per unit mass) Lv =
v = per unit mass) Lv = 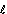 v x m
v x m
Measure 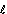 v of steam
Put water in beaker > find mwater > water boils start timing & note reading on balance > mass reduced by specific mass stop timing
v of steam
Put water in beaker > find mwater > water boils start timing & note reading on balance > mass reduced by specific mass stop timing
Assuming negligible heat loss:
IVt = (m2 - m1) 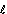 v
v

evaporation & boiling require LV, evaporation: latent heat: 1st taken from liquid (cooling effect) > then from surroundings
TRANSFER OF HEAT
Conduction: transfer of thermal energy w/o any flow of the medium from high > low temp, by lattice vibration and/or electron diffusion (metals)
Lattice vibration: molecules at hot end vibrate vigorously > pass KE to neighbouring molecules by intermolecular bonds > KE ^ > temp ^, occurs in all materials (some non-metals: quartz & certain types of ceramic good conductors using lattice vibration)
Electron diffusion: in metals in addition to lattice vibration, free e-s move randomly bet molecules > heat > e-s (very light) faster KE than normal molecules > hit other molecules > KE ^, temp ^, quick process which makes metals good conductors
conduction: best in solids as molecules close together (hit more often) & intermolecular bonds (able to pass KE to another molecule)
inefficient in liquids and gas: less collision bet molecules & molecules bound together by weaker bonds (liquids)/ no bonds (gas)
poor conductor = insulator, good insulators: gases, good conductors: metals
To investigate heat flow through solids
pin attach by melted wax at end of each rod > insert rods in apparatus > boiling water poured in
conclusions: heat transferred w/o any flow of material (conduction) & it occurs at diff rates
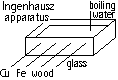
good conductors; metals, certain types of ceramics: for cooking, heat exchanges (laundry- fuel saved)
bad conductors; most non-metals (wood, plastic, glass, air : to minimise heat flow/loss: for handles of pans, irons,table mats, saw dust/ newspapers covering ice, blankets, fibre glass, double glazing (air used), trapping air- insulation
Convection: main method of heat transfer in a fluid (liquid + gas) by means of movement of fluid due to density changes ( which causes convection currents- stream of warm fluid: molecules heated > expand > density decrease > rise > cool molecules, denser > sink to replace former molecules), can't occur in solids as molecules are fixed in place
sea & land breeze (water high heat cap, gain/lose heat), in ponds > form ice, household hot water systems, electric kettles (heating coil at bottom), air conditioners & refrigerators (cool air sinks, warm air rises to be cooled)
Radiation: flow of thermal energy from one place to another by means of electromagnetic waves (esp infra-red rad) (doesn't require in material medium- can occur in vacuum, not like conduction & convection)
-all bodies emit and absorb radiation all the time, depends mainly on temp, texture of surface also affects rate of emission of EM radiation; dull, black surface- godd emitter/ radiator of heat, shiny, bright surface- poor radiator of heat(reflects heat)
(absorb infra-red, energy ^, temp ^, infra-red: causes heat sensation, heat due to infra-red = radiant heat, from Sun, hotter object emits more infra-red)

causes skin cancer: UV rad, Vacuum flasks ;designed to minimised heat loss (by conduction, convection, radiation, evaporation): conduction & convection prevented by vacuum bet walls, stopper reduces convection & evaporation, reduce conduction- glass walls & plastic stopper, shiny, silver walls reduce radiation, white shiny teapots, green house ;infra-red rad enters as shorter wave lengths > emitted w/ longer wave lengths > glass prevents infra-red from escaping > temp higher for plant growth
Waves & Sound
Radioactivity
Measurements In Physics
Forces
Energy
Pressure
Optics
Magnetism
Electrostatic Charging
Electricity
Back to 'O' level notes index
Back to notes index
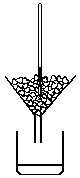







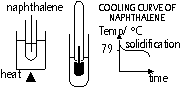


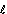 f = per unit mass)
f = per unit mass) 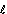 f x m
f x m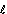 f of ice
f of ice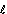 f + micecΔθ2 (Δθ2 = T2)
f + micecΔθ2 (Δθ2 = T2)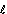 v = per unit mass) Lv =
v = per unit mass) Lv = 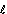 v x m
v x m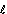 v of steam
Put water in beaker > find mwater > water boils start timing & note reading on balance > mass reduced by specific mass stop timing
v of steam
Put water in beaker > find mwater > water boils start timing & note reading on balance > mass reduced by specific mass stop timing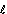 v
v

