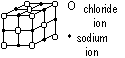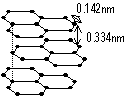GASES, LIQUIDS & SOLIDS part 2
SOLID: giant structure w/ bonding throughout structure (>giant: ionic, metallic, molecular) + simple molecular structure (cov molecules w/ weak intermolecular forcers of attraction)
-GIANT IONIC LATTICE STRUCTURE
consists of cations + anions attracted by strong electrostatic ionic bonds throughout structure
Structure of ionic lattice, NaCl (sodium chloride)
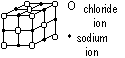
close face-centred closed cubic packing of larger Cl- ions w/ Na+ ion in bet Cl- ions (in 'octahedral holes' = spaces bet Cl- ions), each Cl- ion touches 12 other Cl- ions & 6 Na+ ions
ions held in position by strong ionic bonds
Properties
-High mp + bp: strong ionic bonds need large amt of energy to break
-Hard: strong ionic bonds need large amt of energy to break
-Brittle: ions arranged regularly, when cpd hit > ions of like charge close together > repel > break lattice (ionic crystals usually break/cleave along a regular plane = plane of cleavage due to regular arrangement of ions)
-Don't conduct elec as solids: ions not mobile to carry elec
-Soluble in H2O to become electrolytes: dipole-ion attraction overcomes crystal lattice energy of solid > ions dissociate and cpd dissolves in water
Uses of ionic cpds
some ionic cpds are refractory subs: have very high mp & are heat resistant, MgO (mp: 2900°C), Al2O3 (mp: 2040°C) > don't melt at high temp and don't conduct elec as solids
MgO + Al2O3: line furnaces, Al2O3: in crucibles to contain molten metals
-GIANT MOLECULAR STRUCTURE
consists of covalently bonded atoms throughout structure
-allotropes of C (diamond, graphite + fullerenes) + silicon (IV) oxide, SiO2 (or silica, in sand)
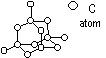
Structure of diamond
carbon atom: sp3- hybridised > tetrahedral units which inter-link to form interlacing network of puckered hexagonal rings, coordination # of C = 4 (no. of other atoms surrounding atom = 4), bond length: 0.154nm, bond angle: 109.5°
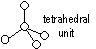
Properties of diamond
-bright & sparkling: all 4 valence e-s used in bonding > no free e-s to reflect visible light energy > not lustrous
-hard: many cov bonds in interlacing network of hexagonal rings
-high mp: > 3000°C, melting (w/o O2) involves breaking of many cov bonds > need large amt of heat energy
Uses of diamond: hardest natural occurring subs > in glass-cutter + rock drills
Structure of graphite
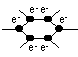
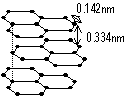
carbon atom sp2-hybridised > trigonal planar > join together to form layers of flat hexagonal carbon rings bet layers- Van der Waals forces, in layer: cov bonds bet C atoms
each C atom has a mobile valence e- (π-e-) which can only move in direction parallel to plane of layer > graphite conducts elec along plane of layer
Properties of graphite
-high mp: 3730°C, many cov bonds must be broken
-soft: layers held in position by only weak VDW forces > layers can slide over each other when force applied (graphite + clay = pencil 'leads')
-electrical conductor: free valence e- of each C atom allows elec to be carried parallel to plane of layer
Structure of fullerenes: allotropes of C60 & C70, sp2-hybridised C atoms form bucky-ball structure w/ central hexagonal rings surrounded by pentagonal rings, possible semi-conductors??
Ceramics: from mixture of giant ionic + giant covalent structures > high mp + heat resistant, MgO, Al2O3, carbides, nitrides & silicates
Properties:- high mp, heat resistant, chemically inert, not soluble in organic solvents, good insulate for elec & heat
Uses:- eg porcelain in crockery, as insulator of large high tension cable, in furnaces & rocket engines
-GIANT METALLIC STRUCTURE
positive metal cores in a sea of mobile valence e-s which attract cores to form metallic bonds
Structure of copper metal
Cu (s): +ve Cu ions occupy fixed lattice position, packed in face-centred cube & surrounded by mobile valence e-s
coordination of Cu2+ ions = 12
Properties
-good conductor of heat & elec: free-moving valence e-s allow this
-high mp & bp: strong metallic bond- strong attraction bet valence e-s + metal cores
-high density: strong metallic bond caused ions to be close packed together
-malleable & ductile: metallic bonds attract ions from all directions > flexible bond
Uses of copper: electric wires, to make alloys (coinage metal: Cu + Ni, brass: Cu + Zn, bronze: Cu + Sn, pewter: Cu + Sn + Pb)
Alloys: mixture of metals, main metal + smaller proportion of other (non-)metals
alloys made to change properties of metals, usually make stronger
Cu: soft & conducts elec well, Zn: harder, doesn't conduct elec as well > Cu + Zn = brass: hard conducts elec well
Fe: strong, but rusts > Fe + Cr + Ni > stainless steel: strong & doesn't rust
-SOLIDS OF SIMPLE MOLECULAR STRUCTURE
3 types: solids consisting of:-
1:atoms w/ weak VDW forces: atoms arranged in face-centred cube, held in position by weak VDW forces, coordination # = 12, v.low mp + bp
2:simple covalent molecules w/ weak VDW forces: molecules arranged in face-centred cube, held in position in crystal lattice by weak VDW forces, coordination # = 12, low mp + bp
3:simple covalent molecules w/ H-bonds: ice, H2O molecules held in lattice by intermolecular H-bonds > diamond-like structure, each molecule: 4 H-bonds
Gases, liquids & solids part 1
Back to 'A' level notes index
Back to notes index