Chem2
EXPERIMENTAL TECHNIQUES
Indicators
-chemicals (usually extracted from plants) that change colour at different pH
pH-measure of acidityacid pH>7, neutral pH=7, alkaline pH<7
methyl orange [red > orange(pH4) > yellow]screened methyl orange [red > grey (pH4) > green]
litmus [red > - (pH7) > blue]phenolphthalein [colourless > - (pH9) > pink]
Criteria of Purity
to prove a substance is pure/impure-melting point (pure-1 m.pt., impure: >1 m.pts.)
-boiling point (pure-1 b.pt., impure-distils over >1 b.pts.)
-chromatogram (pure-1 spot)
Measuring Melting Point
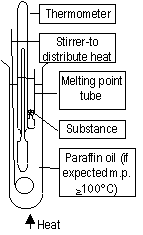
Heat, substance begins to melt > temp. remains constant = melting point, impure: melts over a range ot m.p.'s
Paper Chromatogram (colour-graph)
Chromatography-used to identify mixtures and testing for purity. separate colourless substances: locating agent colours colourless spots. Spots identified by RF value = dist. moved by substance/ dist. move by solvent
Spots separate due to different solubility in solvent
More soluble, moves