QUANTUM PHYSICS
Quantum energy
to explain thermal radn, Planck assumed energy can not be divided into smaller amts and are emitted in discrete 'packets' of energy called quanta (1 packet = 1 quantum)
energy depends on freq, f of source
E = hf
[h: Planck's const = 6.63 × 10-19J (experimentally found)]
some experiments on light show light's -wave nature (interference, diffraction) & -particle nature (photoelectric effect)
Einstein explained particle nature by assuming light (or other em radn) consists of packets of energy called photons (1 proton has 1 quantum of E)
Photoelectric effect
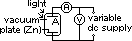
-electron emission from a substance when light is shone on it
electrons emitted reach plate A => galvanometer gives current reading
max KE found by applying stopping voltage,Vs (min V to just stop electrons from reaching plate A => no reading on galvanometer)
eVs = ½mvmax2
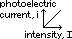
Laws of photoelectric emission
(1) # of photoelectrons emitted per second ∝ intensity of radn
(2) photoelectrons have KE from 0 to max; KE ∝ freq of radn (ie VS is independent of intensity)
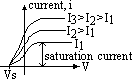
(3) threshold freq, f0 = min freq for emission of electrons to occur (below f0 no electron emission despite intensity & time)
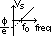
wave theory can't explain (2) & (3)
wave theory suggests radn energy spread wavefront
=> since amt of incident energy on electron is v.small > expect time lapse before electrons have enough energy to escape from surface (experimentally: no time lapse)
Einstein's quantum explanation
-each photon give 1 quantum of E (= hf) to electron
-work function = energy, 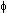 for electron to escape surface
for electron to escape surface
-if hf > work function => excess energy increases KE of electron
Einstein's photoelectric eqn: hf - 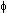 = ½mvmax2
= ½mvmax2
electrons emitted photons absorbed
at threshold freq, f0; Ephoton = hf0 = 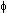 => ½mvmax2 = hf - hf0 = h(f - f0)
=> ½mvmax2 = hf - hf0 = h(f - f0)
½mvmax2 = eVs = hf - 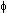 => Vs = hf/e -
=> Vs = hf/e - 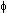 /e [
/e [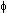 /e = +ve const, h/e = constant > grad for all graphs are equal]
/e = +ve const, h/e = constant > grad for all graphs are equal]
Wave-particle duality
photon of freq, f has E = hf = hc/
Einstein's energy-mass relatn: E = mc2 = hc/ =>
=>  = h/mc [
= h/mc [ : wave-like property, m: particle-like property]
: wave-like property, m: particle-like property]
de Broglie suggested particle w/ momentum, p (=mv) has wave-like properties
de Broglie wavelength,  = h/mc = h/p
= h/mc = h/p
Electron diffraction
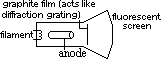
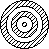
electron beam shows diffraction pattern > wave-like
diffraction pattern also shown by neutrons, protons, alpha particles
 associated w/ electron; ~10-10m (X-ray region)
associated w/ electron; ~10-10m (X-ray region)
diffraction shown when using w.small slit sepn (that of interatomic spacing of crystal)
Evidence for electrons as particles
fixed charge- shown by Milikan's oil drop exp
fixed mass- shown by deflectn of cathode rays by B-filed => circular path of certain radius => therefore e/m same for all electrons; since fixed charge => fixed mass
Spectral lines
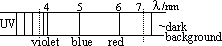
-a spectrum- mixture of wavelengths
electric discharge through hydrogen at low pressure > gas emits particular wavelengths [spectrum made up of lines (visible colours, UV, IR)]
Bohr's quantum explaination
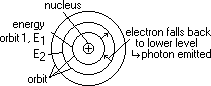
in atom electrons move around nucleus in certain orbits
diff orbit => diff energy (KE + PE) for each electron
electron raised to higher orbital (when collide w/ electron of another atom) > gain E
electron transition: electron falls to lower orbit > lose energy (as photons)
hf = E2 - E1 (certain change in energy due to certain orbits => specific spectral lines => energy quantised)
zero energy when electron at rest outside atom
=> energy at energy levels: -ve (electron 'falls' into atom losing E as em radn)
ground state: electron in lowest energy state available
excited state: electron raised to higher level => empty level below
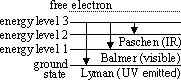
Evidence of energy levels- optical line spectra
-line in optical spectra indicates presence of particular freq (=>  ) of light
) of light
-lines arise from Eloss through transition(s)
-freq n the quantum of 

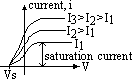
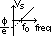
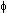 for electron to escape surface
for electron to escape surface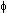 = ½mvmax2
= ½mvmax2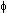 => ½mvmax2 = hf - hf0 = h(f - f0)
=> ½mvmax2 = hf - hf0 = h(f - f0)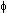 => Vs = hf/e -
=> Vs = hf/e - 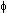 /e [
/e [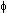 /e = +ve const, h/e = constant > grad for all graphs are equal]
/e = +ve const, h/e = constant > grad for all graphs are equal]
 =>
=>  = h/mc [
= h/mc [ : wave-like property, m: particle-like property]
: wave-like property, m: particle-like property] = h/mc = h/p
= h/mc = h/p

 associated w/ electron; ~10-10m (X-ray region)
associated w/ electron; ~10-10m (X-ray region)


 ) of light
) of light