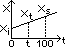Mercury in glass thermometer

thin capillary tube (bore): smaller for higher sensitivity (lower range)
large vol Hg bulb: more expansion per °C => more noticeable increase in Hg thread => lower range
thick glass wall: magnify Hg thread, make thermometer less fragile
thin bulb wall: fast heat conduction > quick response
mercury: expands uniformly, good conductor of heat, coloured, doesn't stick to glass wall
inaccuracy; not uniform bore, bulb shrinks after few years > affects 0 reading, not all Hg at same temp (only some in liquid)
Platinum-resistance thermometer

resistance of Pt increases / temp
t/100 = (Rt - Ri)/(Rs - Ri)
fine Pt wire wound on mica strip & connected to thick Cu leads
a pair of straight identical short-circuited dummy leads enclosed in same silica tube to compensate exactly for changes in R w/ temp of Cu leads to Pt wire
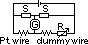
thermometer connected to Wheatstone bridge > adjusted until galvanometer reads zero => RPt = R
very sensitive, can't measure temp at a pt, expensive (Pt), slow response (large heat capacity)
Thermocouple

junctions made of 2 diff metal wires joined together kept at diff temp => small emf prod => I flow
thermo-emf depends on: type of metal, temp diff (emf proportional to temp change)
[emf-temp diff graph: always approx parabolic]
mercury-in-glass thermometer
-39 to 500°C
simple, direct reading, cheap, portable
not v.accurrate, small range, fragile
Pt-R thermometer
-200 to 1200°C
wide range, v.accurate, best for small steady temp diff
not suitable for rapidly changing temp (due to large heat capacity- slow response)
Thermocouple thermometer
-250 to 1500°C
wide range, fairly accurate, can measure -rapidly changing temp, -temp at pt
not as accurate as Pt-resistance thermometer within -200 to 600°C
thermometers based on diff property => diff values for same temp except at fixed pts where most agree by defn
disagreement due to assumption thermometric property varies linearly w/ temp (not true)
Absolute thermodynamic scale
defn depends on theoretical efficiency of perfectly reversible heat engine
theoretical scale => doesn't depend on property of subs
1K = 1/273.16 × triple pt of water [K: kelvin]
triple pt of water, Ttr: temp at which sat water vapor, pure water, ice exists at equilbm = 0.01°C = 273.16K
Ideal gas scale
ideal gas- obeys pV ∝ T exactly => pV/T = const [T in kelvin]
(pV)t/Tt = (pV)tr/273.16 => T = (pV)t/(pV)tr × 273.16
ideal gases don't exist; real gas like ideal gas at low p
temp given by ideal gas: identical to thermodynamic scale
T(K) = pt/ptr × 273.16 [vol = const]
using real gas: by extrapolating to zero => possible to measure ideal gas temp
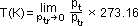
Celsius temp scale
 /°C = T/K - 273.15 [T: thermodynamic temp]
/°C = T/K - 273.15 [T: thermodynamic temp]fixed pts: -273.15K, 0.01°C (Ttr)
| centigrade | thermodynamic | celsius |
| *100°C | 373.15K | 100°C |
| *273.16K | *0.01°C | |
| *0°C | 273.15K | 0°C |
| -273.15 | *0K | *-273.15°C |
Back to 'A' level notes index
Back to notes index