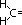
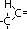
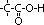 , alkanoic acid
, alkanoic acid
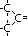
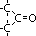 , ketone
, ketone

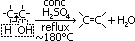
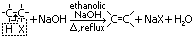
 e-s: e- source for electrophiles > electrophilic addition
e-s: e- source for electrophiles > electrophilic addition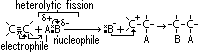
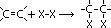
 e- of C=C double bond repel shared pair of e- in Br molecule > dipole induced > electrophilic part of Br molecule(Br+) accepts pair of
e- of C=C double bond repel shared pair of e- in Br molecule > dipole induced > electrophilic part of Br molecule(Br+) accepts pair of  electrons & adds to double-bond C atom > +ve charge carbocation intermediate
electrons & adds to double-bond C atom > +ve charge carbocation intermediate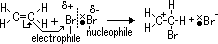
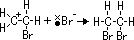
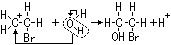
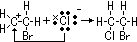
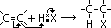
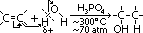
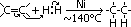
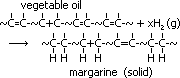
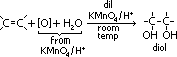
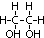 ethane-1,2-diol = glycol: anti-freeze in car radiators, make polyester, terylene
ethane-1,2-diol = glycol: anti-freeze in car radiators, make polyester, terylene
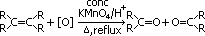
| structural part of alkene | vigorous oxidation product |
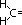
| CO2 + H2O |
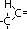
| 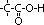 , alkanoic acid , alkanoic acid
|
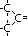
| 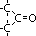 , ketone , ketone
|