| element | atomin no | electronic config | nature |
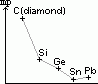
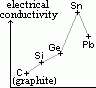
| carbon, C | 6 | 1s22s22p2 | non-metal |
| silicon, Si | 14 | [Ne]3s23p2 | metalloid |
| germanium, Ge | 32 | [Ar]3d104s24p2 | metalloid |
| tin, Sn | 50 | [Kr]4d105s25p2 | metal |
| lead, Pb | 82 | [Xe]4f145d106s26p2 | metal |
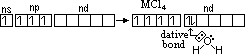
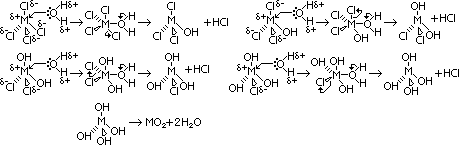
| oxide | structure | type of oxide | thermal stability |
| CO | simple molecular | metal oxide; no reaction w/ acid / alkali | readily oxidised to CO2 |
| SiO | simple molecular | metal oxide; no reaction w/ acid / alkali | readily oxidised to SiO2 in air |
| GeO | predominantly ionic; giant ionic | amphoteric | readily oxidised to GeO2 in air |
| SnO | predominantly ionic; giant ionic | amphoteric | readily oxidised to SnO2 in air |
| PbO | predominantly ionic; giant ionic | amphoteric | stable to heat |
| oxide | structure | type of oxide | thermal stability |
| CO2 | simple molecular | acidic | stable at high temp |
| SiO2 | giant molecular | acidic | stable at high temp |
| GeO2 | intermediate bet giant molecular & giant ionic | amphoteric | stable at high temp |
| SnO2 | intermediate bet giant molecular & giant ionic | amphoteric | stable at high temp |
| PbO2 | intermediate bet giant molecular & giant ionic | amphoteric | decomposes to PbO on heating |
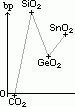
 H= -283kJ/mol
H= -283kJ/mol of M4+(aq)/M2+(aq) system of Ge, Sn & Pb
of M4+(aq)/M2+(aq) system of Ge, Sn & Pb Ge2+(aq),
Ge2+(aq), 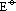 = -1.6V
= -1.6V Sn2+(aq),
Sn2+(aq), 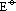 = +0.15V
= +0.15V Pb2+(aq),
Pb2+(aq), 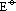 = +1.8V
= +1.8V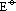 : more +ve from Ge4+ to Pb4+ > +4 state more easily reduced to +2 state
: more +ve from Ge4+ to Pb4+ > +4 state more easily reduced to +2 state