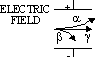
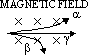
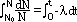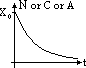| particle | nature | charge | speed (typical) | E (typical) | ionizing effect (ion pairs per mm in air) | penetration (typical) |
| alpha | 2 neutrons + 2 protons | 2e | 0.1c | 10MeV | ~105 | stopped by 0.5mm paper / 50mm air |
| beta | electron | -1e | up to 0.9c | 0.03 to 3MeV | ~103 | stopped by 5mm Al |
| gamma | v.short wavelength em | no charge | c (= 3×108m/s) | 1MeV | ~1 | intensity halved by 100mm Pb |