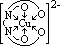| [V(H2O)6]2+ | +2 | violet |
| [V(H2O)6]3+ | +3 | green |
| [VO(H2O)5]2+ | +4 | blue |
| [VO2(H2O)4]+ | +5 | yellow |
| VO3- | +5 | colourless (in alkaline) (in acid: VO3- > [VO2(H2O)4]+) |
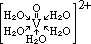
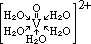
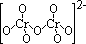
| +6 | dichromate(VI), Cr2O72- | orange (oxidising) |
| +6 | chromate(VI), CrO4- | yellow (oxidising) |
| +3 | chromium(III), Cr3+ | green (most stable) |
| +2 | chromium(II), Cr2+ | blue (reducing) |
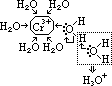
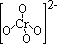
 = +1.33V
= +1.33V| +2 | Mn2+, manganese(II) ion | pale pink (colourless) |
| +3 | Mn3+, manganese(III) ion | dark red |
| +4 | Mn2+, manganese(IV) oxide | black (dark brown) |
| +5 | MnO43-, manganate(V) ion | blue |
| +6 | MnO42-, manganese(VI) ion | green |
| +7 | MnO4-, manganese(VII) ion | purple |
 =+1.52V
=+1.52V =+0.59V
=+0.59V =+1.23V
=+1.23V =-0.13V(since -ve > need heat )
=-0.13V(since -ve > need heat ) =-0.60V
=-0.60V =+0.60V
=+0.60V =+0.04V
=+0.04V =+1.70V
=+1.70V =+0.03V
=+0.03V = +1.81V
= +1.81V = -0.10V
= -0.10V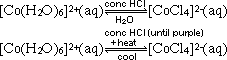
| Fe2+(s) | pale green |
| Fe2+(aq) | pale green [Fe(H2O)6]2+(aq) |
| Fe3+(s) | pale violet (in alum, ammonium iron(III) sulphate) |
| Fe3+(aq) | yellow-brown [Fe(H2O)6]3+(aq) |
| [Fe(CN)6]4-(aq) | yellow |
| [Fe(CN)6]3-(aq) | red |
 = +0.77V
= +0.77V = +0.77V
= +0.77V = -0.56V
= -0.56V = +0.36V
= +0.36V = +0.54V
= +0.54V = +0.77V
= +0.77V = +2.01
= +2.01 = +0.54
= +0.54 = +0.77V
= +0.77V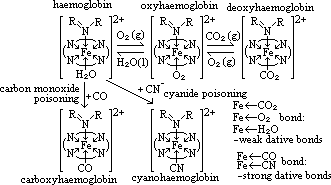
| [Cu(H2O)6]2+ | blue |
| [CuCl4]- | yellow brown |
| [Cu(NH3)4(H2O)2]2+ | deep blue |
| [Cu(EDTA)]2- | light blue |
 = +0.37
= +0.37