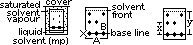CHROMATOGRAHY
chromatography- process of separating components of mixture dpding on partition of component bet 2 phases (mobile & stationary phase)
component / solute will distribute bet stationary phase & mobile phase according to partition law (solute: adsorption on stationary phase, adsorptn: attraction & temp adhering of molecules of gas / liq on solid surface)
solute that distributes more in mobile phase, mp moves faster & separates out first
chromatography: partition / adsorption (according to stationary phase, sp)
partition; paper chromatography, gas-liquid chromatography (GLC)
sp-liq supported on inert solid, mp (eluant): liq /gas
adsorption; column chromatography, thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), gas chromatography (GC) or gas-solid chromatography (GSC)
sp-solid that component are adsorbed on, mp- liq /gas
PARTITION CHROMATOGRAPHY
(1)Paper chromatography
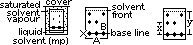
-easy to set up & cheap
-analyse / separate foodstuffs, dyes in food, amino acids from hydrolysis of proteins
sp: layer of water adsorbed on chromatography paper
mp: liq solvent
solvent travels across paper
movement rate of solute along paper dpds on partition coefficient (bet mp & sp)
more soluble in mp > travel faster > separate out first
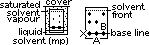
RF value (retention ratio)- ratios of dist moved by solute from base line / dist moved by solvent from base line
diff solute > diff RF value
2-way paper chromatography: (separates components more > easier detectn)
-normal paper chromatography > after initial separatn rotate paper 90° and repeat chromatography
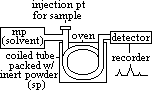
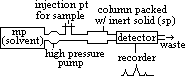
(2)Gas-liquid chromatography (GLC)

-petroleum industry (analyse oil slicks), analyse exhaust fumes, pesticides in crops foodstuffs, water samples, air samples, blood, urine
mp: inert gas / carrier (He/N2)
sp: inert long-chain hydrocarbon (involatile) oil supported by surface of inert solid packed in column (longer than in HPLC)
sample injected into GLC > vapourised > vapour passes through packed column by mp (helium) [sample analysed needs to be in vapour form for analysis (can be liq or gas at rtp)]
column maintained at cont temp in oven
component separate dpding on volatility & solubility on oil (less volatile & more soluble > retained longer in column)
recorder detects components, by retention time or by spectroscopic analysis, and represents them as peaks
chromatogram diagrams
-larger peak > larger amt of subs
-elution time ~ in order of bp of subs
ADSORPTION CHROMATOGRAPHY
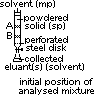
(1)Column chromatography
-analyse purity of medicine, separate colouring in food (usually separates rel large quan)
sp: solid adsorbent (alumina, Al2O3; silica, SiO2) in packed column
mp: solvent (eluant)
mp moves down, over the solute > solute distributes bet mp & sp
rate of movement down column dpds on partition coefficient
more soluble in mp & less adsorbed in sp > move down faster > separates out faster
(2)Thin layer chromatography (TLC)
-analyse food dyes, poisons (in autopsy), amino acids in blood, pesticides in crops
sp: solid adsorbent (silica gel / alumina, Al2O3) coated on inert plastic / glass plate
mp: liq solvent
solvent moves across solute > solute distributes itself bet mp & sp according to partition coefficient
more soluble in mp & less adsorbed in sp > move faster separates out first
(3)High-performance liquid chromatography (HPLC)
mp: liq solvent
sp: inert solid (siloxane) packed in column
sample forced through inert solid at high pressure
less soluble in liq, more adsorbed on solid retained longer
ELECTROPHORESIS
analysis & separatn of charge particles / ions based on movement in electric field (use adsorbent eg filter paper in buffer / agarose gel on plastic plate)
+ve ion move to –ve electrode, -ve ion move to +ve electrode
sheet soaked in mixture > high pd applied across sheet > subs move in diff direction & separate dpding on mass on subs
Applications of electrophoresis
-identify biochemical materials (blood, proteins) which are usually charged (amino acids/ zwiterrions can be separated)
-genetic finger-printing
amino acids exist as zwitterions
acidic conditns (excess H+): exist cations > move to -ve electrode
alkaline conditns: exist as anions > move to +ve electrode
SOLUBILITY OF GASES
solubility of gas in solvent = vol of gas (cm3), measured at 0(C & 1atm, that dissolves in solvent per cm3 at a temp, under partial pressure of 1atm of gas
factors affecting solubility: -nature of gas, -temp, -pressure
Nature of gas
more soluble if gas reacts chemically w/ water (NH3, HCl, SO2, CO2)
-reactn due to polarity of molecule (CO2: non-polar molecule, but has polar bonds)
non-polar molecules do not react w/ water > solubility less
Temperature
gas dissolves in solvent > exothermic
gas + solvent ( sol, (H= -ve
high temp: eqlbm shift to left (Le Chateliers prinicple) > less soluble
Pressure of gas
Henry's law: mass of gas which dissolves in given vol of solvent, at given temp, is proportional to pressure of gas / partial pressure of gas in gas mixture
m ∝ pgas
m ∝ conc gas dissolve, [gas]
=> [gas] ∝ pgas
=> [gas]/ pgas = kh = Henry's law const
Validity of Henry's law
-cont temp (> const solubility)
-gas & solvent don't react chemically
-solutn formed on dissolving must by dilute
-pressure not too high & temp not too low (gas characteristics deviate)
Applications of Henry's law
-conc of oxygen breathed in (during surgery)
-divers & mountain climber control O2 & N2 levels in blood
-removing dissolved gases in liq by applying suction / vacuum above liq
-dissolved air in water (for fish)
Back to 'A' level notes index
Back to notes index